39 what is an open label study
Open-label versus double-blind placebo treatment in irritable bowel ... The study also examines possible genetic and psychological predictors of OLP and seeks to better understand participants' experiences with OLP and DBP through a series of extensive interviews with a randomly selected subgroup. ... and episodic migraine, have demonstrated that individuals receiving open-label placebo (OLP) can still experience ... Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered.
National Center for Biotechnology Information by GJ Taylor · 2005 · Cited by 55 — Open label extension studies typically follow a double blind randomised placebo controlled trial of a new drug. At the end of the double blind ...

What is an open label study
Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years). What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. Can I create an open-label study or an open-label period? Yes. You can create open-label periods, including open-label extensions, and an entirely open-label study. Parent topic: Kit and dispensation FAQs (for study designers)
What is an open label study. Effects of open-label placebos in clinical trials: a systematic review ... Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze the... PDF What Are Open-Label Extension Studies For? factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation. Proactive infliximab optimisation using a pharmacokinetic dashboard ... The aim of this randomised, controlled, multicentre, open-label trial is to evaluate the efficacy and safety of a proactive TDM combined PK dashboard-driven infliximab dosing compared with standard of care (SOC) dosing in patients with moderately to severely active CD.
What is the optimal dose of escitalopram for the treatment of obsessive ... A naturalistic open-label study Int Clin Psychopharmacol. 2011 Sep;26(5):284-90. doi: 10.1097/YIC.0b013e32834a5c09. ... This study explored the efficacy and tolerability of very high doses (maximum dose of >40 mg/day), high doses (maximum dose of 25-40 mg/day), and standard doses (maximum dose of ≤20 mg/day) of escitalopram (ESC) as an anti ... Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study. A Phase I, Open-label Study to Assess the Safety and Efficacy of Poly ... This is an open-label, phase I pilot study. A total of 10 patients will be recruited in National Taiwan University Hospital between October, 01, 2021 and December, 31 2023. All examinations and treatment will be performed at National Taiwan University Hospital. The inclusion criteria are Child-Pugh classification A, unresectable HCC patients ...
Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA … 25.2.2020 · None (Open Label) Primary Purpose: Prevention: Official Title: Phase I, Open-Label, Dose-Ranging Study of the Safety and Immunogenicity of 2019-nCoV Vaccine (mRNA-1273) in Healthy Adults: Actual Study Start Date : March 16, 2020: Estimated Primary Completion Date : November 22, 2022: Estimated Study Completion Date : November 22, 2022 A Long-Term, Open-Label Study to Evaluate the Safety and Tol ... Orion was a multicenter, open-label study, conducted from October 7, 2011, to May 18, 2017. Patients were screened for eligibility at the last visit of their respective parent study, which was defined as the baseline visit for Orion. Eligible patients entered a 52-week, open-label treatment phase (visits at weeks 1, 2, 4, 8, 14, 20, 26, 32, 38 ... Meaning of "Open Label Pilot study"..?? - SAS Support Communities Open label is a term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. Open label study - Medical Dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
Open-Label Trial | NIH - HIV.gov Open-Label Trial Audio Your browser does not support the audio element. 530.mp3 A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term(s) Clinical Trial Double-Blind Study Print Print this term Download Glossary
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key...
Open-label study - definition of Open-label study by The Free Dictionary Open-label study synonyms, Open-label study pronunciation, Open-label study translation, English dictionary definition of Open-label study. n. pl. stud·ies 1. a. The effort to acquire knowledge, as by reading, observation, or research: The study of language has overturned many misconceptions.
Can Open-Label Placebos Be Effective Medical Treatments? The effectiveness of open-label placebos. Dr. Jeremy Howick, a clinical epidemiologist, reviewed five research studies involving a total of 260 patients and found that open-label placebos often yield the same positive results as reflected by the placebo effect. These studies involved a variety of conditions, including irritable bowel syndrome ...
NCI Dictionary of Cancer Terms A type of study in which both the health providers and the patients are aware of the drug or treatment being given. Search NCI's Dictionary of Cancer Terms.
What is the best critical appraisal tool to for a single-arm open-label ... To perform a quality assessment of included studies for an meta analysis consist of retrospective studies . There are many tools as Cochrane items, the most reliable tools, but if we apply this ...
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
Open-label Definition & Meaning - Merriam-Webster The meaning of OPEN-LABEL is being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers ...
Open Label Study Using OsteoProbe System - Full Text View ... Open Label Study Using OsteoProbe System (OP) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Medical Definition of Open-label - MedicineNet Reviewed on Privacy & Trust Info Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving.
(PDF) What is an open label trial? - ResearchGate Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may...
Can I create an open-label study or an open-label period? Yes. You can create open-label periods, including open-label extensions, and an entirely open-label study. Parent topic: Kit and dispensation FAQs (for study designers)
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years).









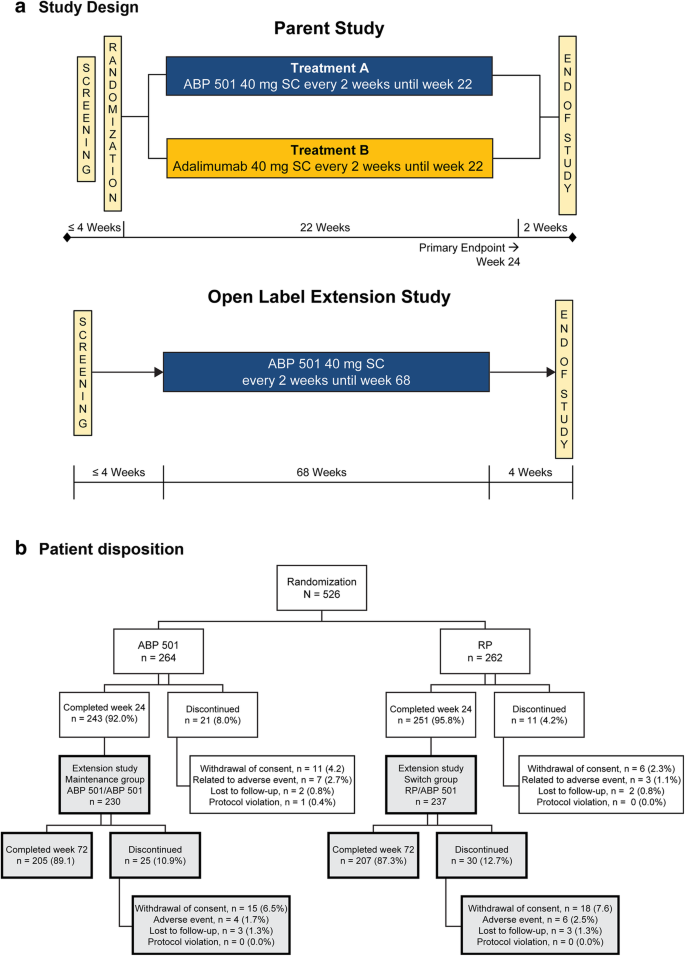

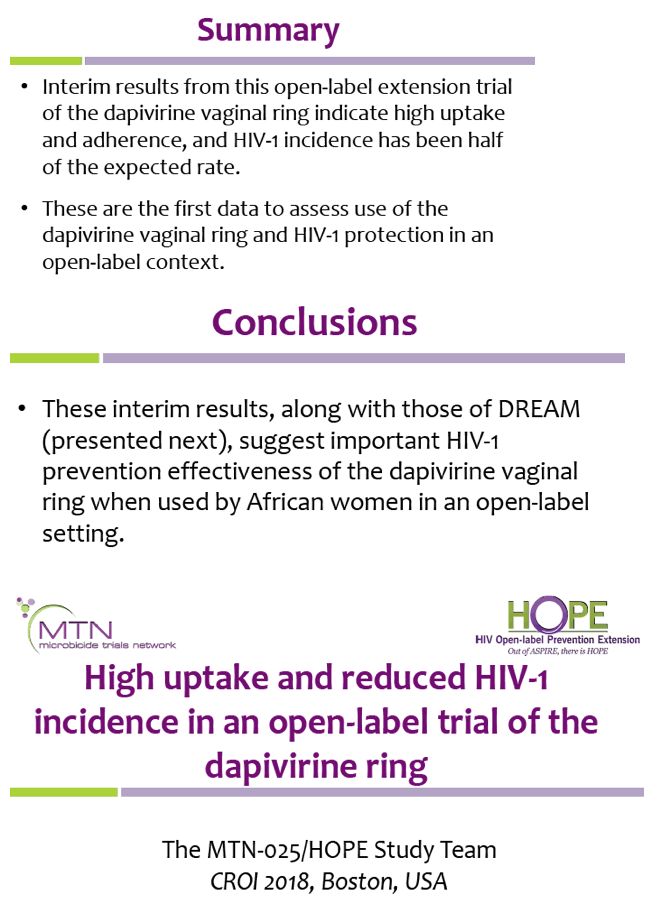














![PDF] Safety of a novel gel formulation of clindamycin ...](https://d3i71xaburhd42.cloudfront.net/0916a0bbf4b4dc0abf87c160baa6097e46751a8f/2-Table1-1.png)

![PDF] ZINC HYALURONATE IN THE TREATMENT OF DIABETIC FOOT ...](https://d3i71xaburhd42.cloudfront.net/fc5a851ceb2630c27c4ef0786beda8efd5be5fae/3-Table1-1.png)




Post a Comment for "39 what is an open label study"