41 chemical equation labels
4.1 Writing and Balancing Chemical Equations - Chemistry First, write the unbalanced equation. N2 +O2 N2O5 (unbalanced) N 2 + O 2 N 2 O 5 ( unbalanced) Next, count the number of each type of atom present in the unbalanced equation. Though nitrogen is balanced, changes in coefficients are needed to balance the number of oxygen atoms. What are the Parts of a Chemical Equation? - Life Persona The steps followed to write a chemical equation are: - Reagents and reaction products are identified and noted. - The formula or symbols of the reagents are written on the left side with a '+' sign between them. - The formula (s) of the products are written on the right side with a '+' sign between them.
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Start by writing the chemical equation in terms of the substances involved: C 2 H 6 + O 2 → CO 2 + H 2 O We have two carbon atoms on the left, so we need two carbon dioxide molecules on the product side, so that each side has two carbon atoms; that element is balanced.
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Chemical equation labels
Chemical Equation Balancer The equation will now look like this: 4Na + O 2 = 2Na 2 O This equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. Example #2 (Complex) P 4 + O 2 = 2P 2 O 5 This equation is not balanced because there is an unequal amount of O's on both sides of the equation. Chemical Equations - Let's Talk Science A chemical equation is a way to represent a chemical reaction. The reactants are given on the left hand side and the products are given on the right hand side. Instead of an equal sign, an arrow is drawn between the reactants and the products. It is important to note that the arrow does not mean the same thing as an equal sign. What are Chemical Equations? Detailed Explanation, Examples List some Examples of Chemical Equations. A few examples of chemical equations are listed in bulleted text below. PCl5 + 4H2O → H3PO4 + 5HCl SnO2 + 2H2 → 2H2O + Sn TiCl4 + 2H2O → TiO2 + 4HCl H3PO4 + 3KOH → K3PO4 + 3H2O Na2S + 2AgI → 2NaI + Ag2S How do you write chemical formulas?
Chemical equation labels. Chemical equation - Wikipedia A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction. Solved 1. Write a balanced thermochemical equation with | Chegg.com Chemistry questions and answers 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3. How to write chemical formula with branching and with labels for each ... Here is a solution along the lines of what you were doing for giving equation style labels to tikz diagrams. It defines an option eqn-label for a node which puts an equation number on the right hand side of the page on level with the current node. This uses the current page node so you need to include the remember picture option in the tikzpicture options. Chemical Nomenclature and Chemical Formulas - Owlcation Common Acids HBrO 3 - Bromic acid HlO 3 - Iodic acid HClO 3 - Chloric acid HBO 3 - Boric acid HMnO 3 - Manganic acid H 2 SO 4 - Sulfuric acid H 3 PO 4 - Phosphoric acid H 3 AsO 4 - Arsenic acid Note that all acids above have names ending in 'ic' attached to the stem of the name of the nonmetal.
Factor-Label Method in Chemistry: Definition, Examples & Practice ... The factor-label method in chemistry is a method for converting units of measure from one kind into another using factors made from the equalities between the units. Learn more about the definition... How do you Write a Chemical Equation? - A Plus Topper The products of reaction are written in terms of their symbols or molecular formulae on the right-hand side of the equation. A plus (+) sign is added between the formulae of the products. In between the reactants and the products an arrow sign ( ) is inserted to show which way the reaction is occurring. A + B C + D For the chemical equations shown below, label each reactant as either ... For the chemical equations shown below, label each reactant as either acid or base, and each product as either conjugate acid or conjugate base according to the brønsted-lowry definition. drag each label to the appropriate target. ... Advertisement jayilych4real jayilych4real An acid is a chemical compound which helps to increase the amount of ... Chemical Equations - GitHub Pages Write a balanced chemical equation that summarizes this reaction. Balance each equation. MgCl 2 + K → KCl + Mg C 6 H 12 O 6 + O 2 → CO 2 + H 2 O NaN 3 → Na + N 2 (This is the reaction used to inflate airbags in cars.) Balance each equation. NH 4 NO 3 → N 2 O + H 2 O TiBr 4 + H 2 O → TiO 2 + HBr
How do you label a chemical equations? - Answers What are chemical equations? chemical equations are a formal algebra that permits the calculation of chemical reactions. Chemical Equation | Reactants And Products In Chemical Reactions We will first take the maximum number of atoms present on either side of the reaction, that we can find on product side. (Oxygen = 4). Then we multiply the number of oxygen atoms on reactant side by 4, such that the number of oxygen atoms on both sides of the reaction is balanced. The equation becomes F e + 4 H 2 O → F e 3 O 4 + H 2 Chemical formulae - LaTeX Cookbook Chemical formulae and equations have a different style compared to mathematical formulae and equations. For example: Letters mean atomic symbols and are written upright, unlike italic math variables. Numbers are commonly used in subscripts, indicating the number of atoms. We use a lot of subscripts and superscripts, they should be aligned properly. How to Write a Chemical Equation (with Pictures) - wikiHow The final chemical formula for dinitrogen hexafluoride is N 2 F 6. 6. Practice with some examples. When first learning chemistry, there is a lot of memorization involved. It is kind of like learning a new language. The more examples you practice with, the easier it will be to decipher chemical formulas in the future and learn the language of ...
How would you label each formula in the chemical equation below as ... Fe + S -> FeS | Socratic How would you label each formula in the chemical equation below as either a reactant or a product? F e + S → F eS Chemistry Chemical Reactions Chemical Reactions and Equations 1 Answer anor277 Jan 23, 2017 We designate the reactants as written........ Explanation: The reactants are on the LEFT HAND SIDE of the equation.
How to write a chemical formula? - TeX - Stack Exchange In order to produce the following output involving a chemical formula. I can attempt to write the chemical formula as a mathematical formula: \documentclass {report} \usepackage {amsmath} \begin {document} As the scintillator $\text {PbWO}_ {\text {4}}$ crystals are used. \end {document} However, this method is a brute force when approaching a ...
How to Number or Label Equations in Microsoft Word Open your document and select your first equation. On the References tab, click "Insert Caption" from the Captions section of the ribbon. In the Caption pop-up window, select "Equation" next to Label. This sets both the word and the number as the caption. Optionally, select a Position for the caption and click "OK" to apply the caption.
What Are Chemical Equations? - ThoughtCo This can be seen in the following equation: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid. Another symbol you may see is (aq), which means the chemical species is in water — or an aqueous solution. The (aq) symbol is a sort of shorthand ...
Label the chemical equation. 2 Mg(s) + O2(g) → 2 MgO(s) Label the ... Given the chemical equation: 2 Mg(s) + O2(g) → 2 MgO(s) 2 molecules of Magnesium + Oxygen gas (Reactant) → ( yield) 2 molecules of magnesium oxide (product) 2 in Mg and MgO (coefficients) 2 in Oxygen gas (subscript) Solid (s) - physical state of Reactant Mg. Gas (g) - physical state of Reactant O2. Solid (s) - physical state of product 2MgO
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... This is an example of a chemical equation, which is a concise way of representing a chemical reaction. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow.
Examples of Balanced Chemical Equations - ThoughtCo To write a balanced equation, the reactants go on the left side of the arrow, while the products go on the right side of the arrow. Coefficients (number in front of a chemical formula) indicate moles of a compound. Subscripts (numbers below an atom) indicate the number of atoms in a single molecule.
What are the 6 Elements of a GHS Label? - Computype Pictograms 1. Product Identifier/Ingredient Disclosure This component of the GHS label is typically placed in the top left-hand corner of the label, and it identifies the hazardous chemical or ingredient that is in this product. It can state the name, code number, or batch number. This allows for the chemical to be confidently identified. 2.
What is a Chemical Equation? - Definition & Examples A chemical equation provides information about the correct proportions of ingredients that are needed to make new substances in chemical reactions. To make a large glass of lemonade, use 1 1/2 ...





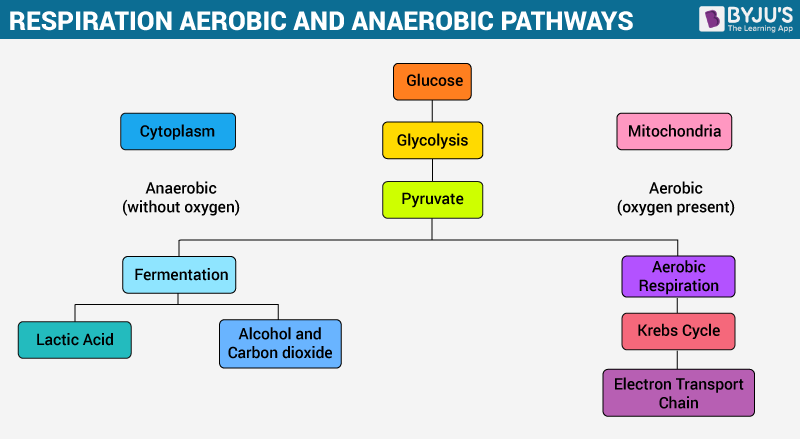


Post a Comment for "41 chemical equation labels"